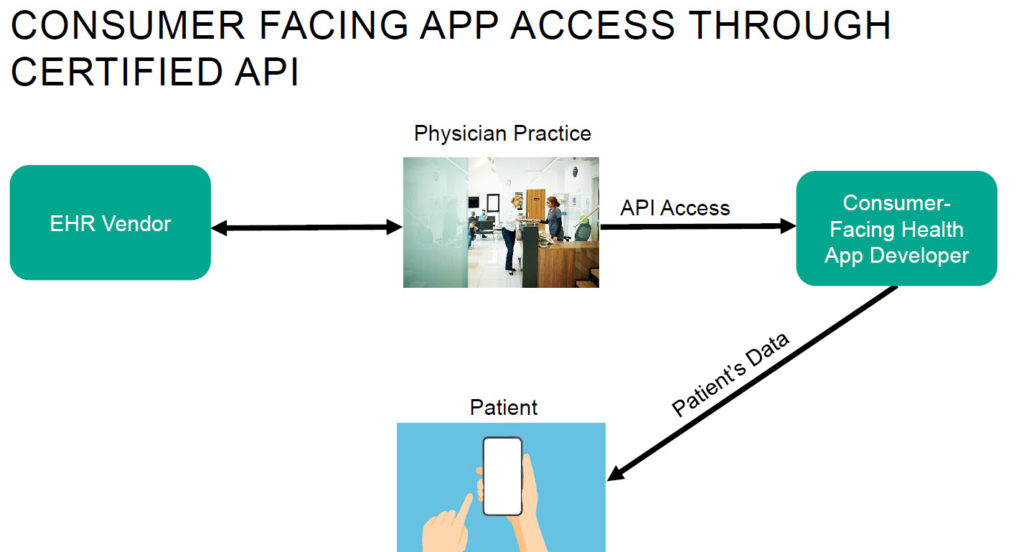
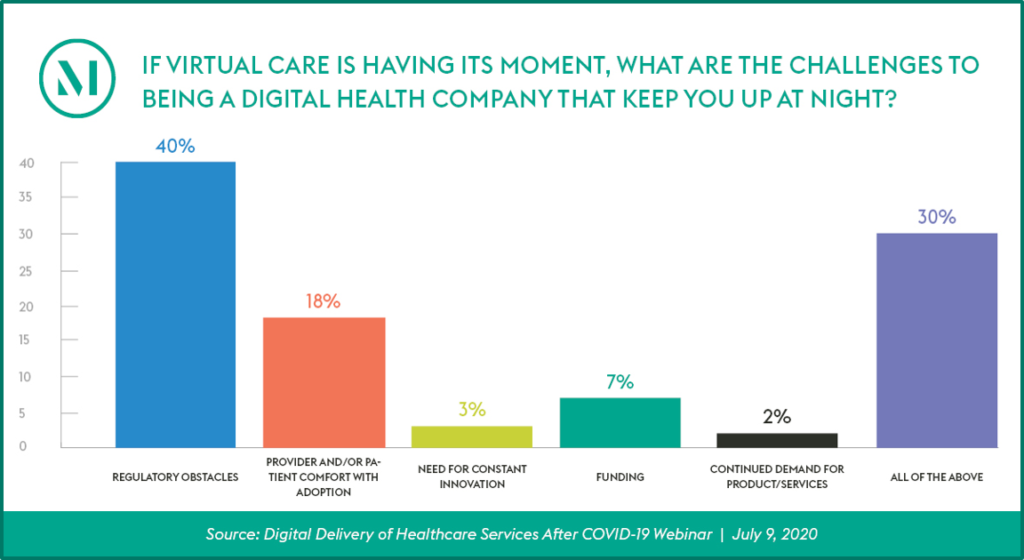
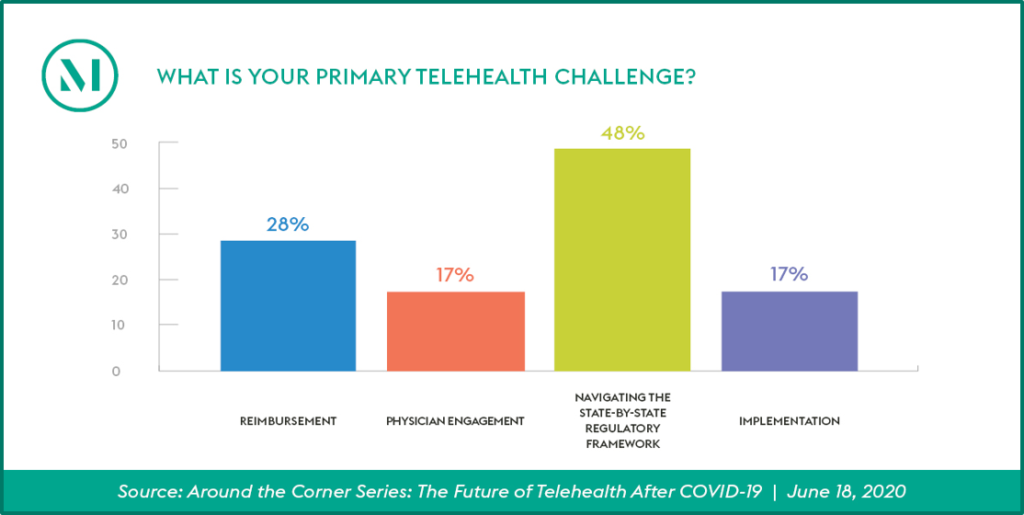
The US Department of Justice and the US Department of Health and Human Services Office of Inspector General recently announced a significant healthcare fraud takedown involving $4.5 billion in allegedly false and fraudulent claims involving telehealth. The allegations involved telehealth executives paying healthcare providers to order unnecessary items and services, as well as payments from durable medical equipment companies, laboratories and pharmacies for those orders. While the alleged conduct is not representative of the legitimate and crucial telehealth services offered by the vast majority of healthcare providers, the government’s continued focus on telehealth arrangements, combined with the ongoing expansion of coverage for telehealth services, provides an important opportunity for healthcare providers to evaluate their telehealth service offerings and arrangements and to further enhance their related compliance activities.
In Depth
On September 30, 2020, the US Department of Justice (DOJ) issued a press release describing the largest national healthcare fraud and opioid enforcement action in the DOJ’s history (the Takedown). The Takedown involved coordination with the US Department of Health and Human Services Office of Inspector General (OIG) and other federal and state law enforcement agencies, and resulted in cases against more than 345 defendants in 51 judicial districts. The government charged the defendants with participating in healthcare fraud schemes involving more than $6 billion in alleged losses to federal health care programs, with the vast majority of alleged losses ($4.5 billion) stemming from arrangements involving alleged “telefraud.”
According to the DOJ press release, a recently announced National Rapid Response Strike Force led the initiative focused on telehealth. The National Rapid Response Strike Force is part of the Health Care Fraud Unit of DOJ’s Criminal Division Fraud section, and its mission is to “investigate and prosecute fraud cases involving major health care providers that operate in multiple jurisdictions, including major regional health care providers operating in the Criminal-Division-led Health Care Fraud Strike Forces throughout the United States.”
Background
In recent years, the government has increasingly focused on alleged healthcare fraud schemes involving telehealth services. In connection with the Takedown, OIG issued a fact sheet and graphic highlighting the increase in “telefraud” arrangements leveraging “aggressive marketing and so-called telehealth services.” The individuals charged in the Takedown included telehealth company executives, medical providers, marketers and business owners who allegedly used telemarketing calls, direct mail, and television and internet advertisements to collect information from unsuspecting patients.
Many of the cases involved telehealth executives who allegedly paid healthcare providers to order unnecessary durable medical equipment (DME), genetic and other diagnostic testing, and medications, either without any patient interaction or with only a brief phone call. The government alleged that the arrangements involved kickbacks to telehealth executives after the DME company, laboratory or pharmacy billed Medicare or Medicaid for items and services that the government asserts were often not provided to beneficiaries or were “worthless to patients . . . and delayed their chance to seek appropriate treatment for medical complaints.”
DOJ provided a [...]
Continue Reading
read more

 Subscribe
Subscribe