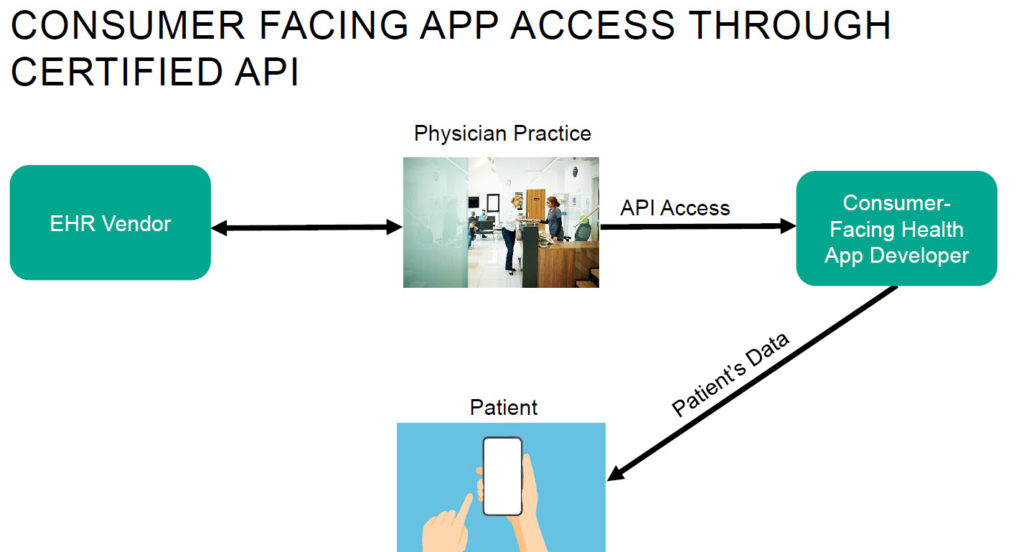
Telehealth’s state-by-state regulatory patchwork means that healthcare providers must navigate a variety of regulations that govern which types of care can be provided by virtual means, and even what modalities can be used in different care settings.
Our new interactive map explores the standards and requirements that physicians and nurse practitioners must follow when prescribing non-controlled substances or ordering tests via a telemedicine encounter in all 50 states and the District of Columbia. Key issues addressed in the survey include:
- In what states are asynchronous solutions permitted?
- What are state rules governing prescriptions when a physician-patient relationship does not exist prior to the telehealth encounter?
- What are state rules on prescribing via audio-visual encounters or audio-only encounters?
- Under what state regulations can a questionnaire be sufficient to create a physician-patient or advance practice registered nurse-patient relationship?
read more

 Subscribe
Subscribe