The idea of keeping people healthy at home has become more relevant than ever during the COVID-19 public health emergency. The expansion of telemedicine during the pandemic is expected to serve as a catalyst that will permanently change the way providers deliver care and patients engage with their health. Joined by leaders from Cricket Health, Livongo and BehaVR, we discussed factors driving the shift towards expanding digital delivery of healthcare services and the challenges – technological, regulatory and cultural – that impact such expansion. Click here to listen to the webinar recording, and read on for highlights from the program.
To learn more about the “Around the Corner” webinar series and attend an upcoming program, click here.
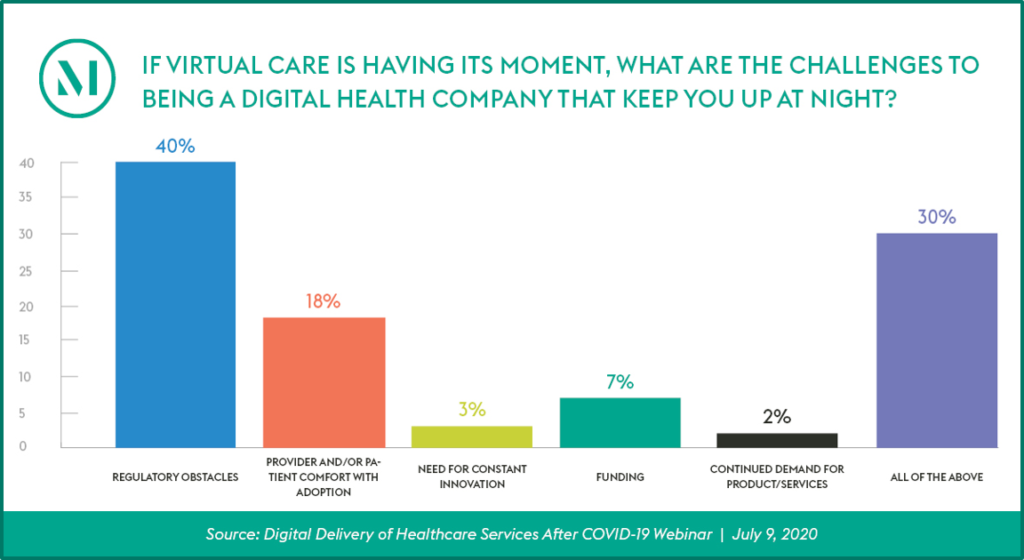
Audience Perspective
Program Insights
- A redoubled focus on preventative care will be key to bring about effective digital health delivery. The current US healthcare delivery system, built mainly on reimbursable, episodic care, is consistently indicted for being a “sick care” system, not a “healthcare” system. Patients, especially patients with chronic healthcare conditions such as diabetes, hypertension, behavioral health and acute kidney disease, need constant, real-time support and guidance, and need their providers to have access to accurate, actionable information to manage these conditions between real-time encounters. Digital health will play a vital role in this effort.
- New care modalities open the door to structural changes, which will need to keep pace with the healthcare system. How emerging care modalities are integrated into and affect the healthcare system are still in development, and raise a variety of concerns, from staffing and technology needs to privacy safeguards. As the healthcare system adapts to these changes, the regulations that govern care delivery, licensing, and accreditation will need to adjust as well.
- Positive regulatory changes have been implemented during the pendency of the national pandemic emergency, but those or similar regulatory changes must continue, and gain momentum and reach, for lasting changes to occur. The actions taken by regulators during the COVID-19 public health emergency show that the government can swiftly respond to new ideas and paths to care. However, these actions are temporary, and it will take time to implement lasting change. While there is an appetite to make some common-sense changes permanent, other areas, such as multi-state professional licensing, will likely take more time due to their complexity.
- Reimbursement models based around episodic care are a major hurdle to the adoption of on-going remote monitoring and other digital health tools. Panelists agreed that when reimbursement structures are aligned with value-based care, such that providers are reimbursed for the outcomes and on-going care management they provide, digital health tools become a critical part of the provider’s toolbox. In [...]
Continue Reading
read more

 Subscribe
Subscribe



